To Grow One Must Change: The Case of Contract Research Organizations


The Secret Heroes of Pharma
Pharmaceutical, biotechnology and medical device companies are responsible for creating and testing new medicines. As such, these companies are the “sponsors” of running clinical trials: carefully designed studies that make sure new drugs are safe and effective for real patients.
However, testing a single drug can take more than a decade and may involve thousands of patients across many hospitals and countries. Because of this scale, the average cost of bringing one new drug to market can exceed one billion dollars. Most companies, especially smaller biotech firms, do not have enough staff, labs, or experience to manage every task required in these trials.
To handle these challenges, many drug developers outsource part or all of the clinical trial process to companies specifically designed to conduct trials: Contract Research Organizations (CROs). CROs take on a wide range of responsibilities. Before a trial begins, they help design the study, create testing plans, and file documents with research ethics boards and government regulators. They identify and recruit the best research centers and doctors, negotiate contracts, and set up reporting systems. Once the trial starts, CROs manage patient enrollment, monitor trial progress, ensure hospitals and doctors follow the rules, and collect and analyze incoming data. When the study ends, CROs organize and review the results, prepare scientific reports, and help sponsors present their findings to regulators for approval.
Because these organizations are so central to modern drug development, the CRO industry is growing rapidly. Analysts estimate the global CRO market will reach nearly $93 billion by 2026 and could climb to more than $138 billion by 2031, expanding at an annual growth rate of more than eight percent. This growth reflects the increasing role CROs play in helping bring new therapies to market, especially as science and clinical trials become more complex.
As science grows more complex, CROs must adapt to keep pace. Three trends are beginning to shape the next phase of the industry, including investment in precision medicine infrastructure, efforts to address persistent clinical trial bottlenecks and the integration of AI into research operations: areas that warrant closer attention from investors.
Becoming More Precise
One of the biggest changes happening in healthcare today is the rise of precision medicine. Doctors intend to shift away from the traditional one-size-fits-all approach of medicine, where treatments of a disease are designed for an “average” patient. Instead, doctors hope to be more effective by assigning precise treatments based on patients’ unique genes, environment and lifestyle.
Advances in genetic technology are making precision medicine an incoming reality. New genome-sequencing tools now allow scientists to analyze a person’s DNA much faster and more cheaply than in the past. For example, recent studies show that whole-genome sequencing can now diagnose certain genetic diseases in hours instead of weeks, which matters enormously for critically ill infants and children. Companies are commercializing ultra-rapid sequencing systems. This is supported by nation-wide biobank programs — in which volunteers donate DNA, medical records, and lifestyle information — that collect massive amounts of genetic data to help researchers uncover genetic risk factors, predict disease progression and develop targeted therapies. At the same time, gene therapy and editing is becoming more prominent as a form of treatment: 49% of therapies in development are gene-based treatments. Some of the most dramatic examples involve children with rare genetic diseases who are treated with personalized gene-editing therapy using CRISPR (clustered regularly interspaced short palindromic repeats) technology and recover in months. As more breakthroughs like these occur, regulatory support (from the FDA) is growing at an unprecedented pace. Analysts estimate that the global precision medicine market will grow to approximately $537 billion by 2035, quadrupling what it is worth today.
Many CROs today operate with traditional trial workflows: standardized data collection, routine monitoring and broad patient recruitment strategies. But precision medicine requires something much more advanced: the ability to run genomic sequencing at scale, integrate genetic and clinical data in real time, manage secure cloud-based analytics and identify highly specific patient subgroups using biomarkers (measurable indicators like genes or proteins that indicate something about disease). CROs that work to attain these abilities, whether through partnering with sequencing companies or investing in genomic datasets, can become indispensable partners in personalized medicine, capturing a large share of the industry’s next decade of growth.
Patients Wanted
Sponsors and CROs will furthermore find structural bottlenecks in today’s clinical trial system. The traditional clinical trial process, where researchers recruit patients at a limited number of hospitals and run studies mostly in person, is becoming harder to sustain. As the rush of COVID-19 trials winds down, overall patient enrollment has fallen back to where it was a decade ago and is expected to remain nearly flat through 2028.
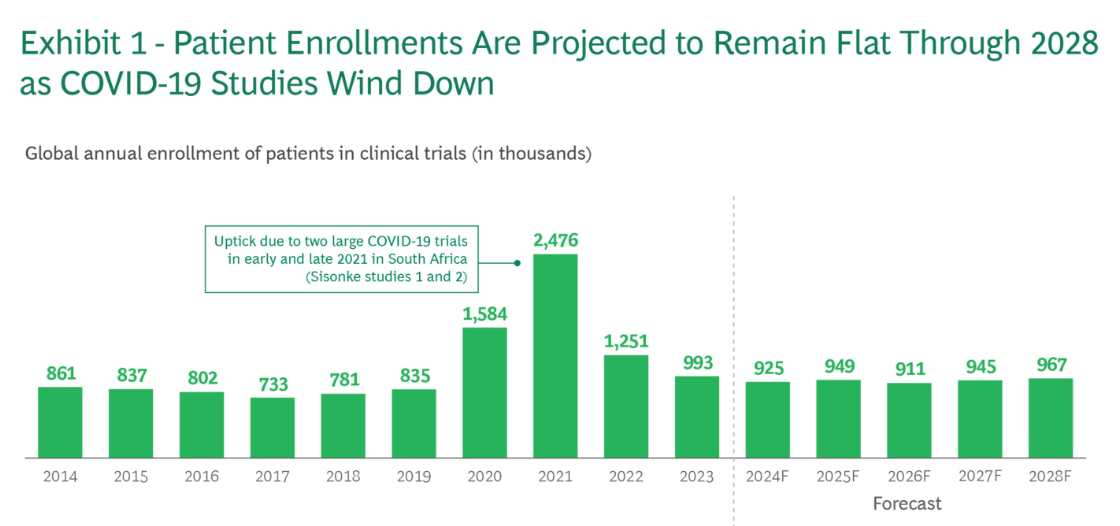
Source: BCG
These problems are magnified by the rapid growth of new medicines entering development, especially in cancer. The number of oncology programs in the pipeline increased by about 13% per year from 2018 to 2022, which sounds like great progress. But if drug development continues to grow at this pace, analysts estimate that the demand for trial participants could more than triple by 2032 — far more than the current system can support. This mismatch is serious: most clinical trials already fail before they finish, not because treatments are unsafe, but simply because they cannot enroll enough patients. Industry data shows that 60% to 70% of trials targeting specific diseases never meet their enrollment goals, and only 5% to 8% of eligible patients in the US ever participate in a study at all, a number that has barely budged in years.
The pandemic briefly demonstrated that there is another way to run clinical studies. During COVID-19, researchers were forced to drop many long-standing rules and began experimenting with new models. Patients could sign consent forms online, ship samples from local labs rather than traveling long distances, and meet doctors over video instead of in person. What had been debated for years suddenly became real in a matter of months: clinical trials did not have to be tied to a hospital building.
As a result, the American Medical Association and the National Institutes of Health advocate for more patient-centric approaches to clinical trials. Instead of beginning with a physical hospital site and hoping the right patients show up, these models identify patients where they already are using electronic health records, insurance data and wearables. Once the right people are located, data can be conveniently collected from patients by having them use mobile apps, wearable devices or provide samples at nearby clinics instead of traveling hours to academic hospitals. These models could generate as much as $8 billion in revenues for CROs, driven by faster and more efficient trial execution.
CROs already manage the logistics of trials, but the industry’s future leaders will be those that adopt remote monitoring, digital enrollment, flexible data collection, and patient-centric technology at scale. By building platforms that solve enrollment barriers, streamline oversight, and support decentralized models, CROs can help fix the bottlenecks of trials that are slowing drug development trials. CROs that succeed will grow into a central role in reshaping how medicine is tested in the years ahead.
Yes, You Can Use Chat
CROs already operate in a predictable, fee-for-service business, but margins tend to be thinner compared with running full clinical programs. That means automation and AI are no longer optional — they are becoming necessary to stay competitive and reduce costs. At the same time, the cost of running trials keeps rising. Since 2014, per-trial costs have climbed steadily, with single-country trials increasing nearly three percent per year, and multinational trials rising almost five percent per year. With budgets tightening and trials getting more expensive, CROs are looking to technology to work faster and more efficiently.
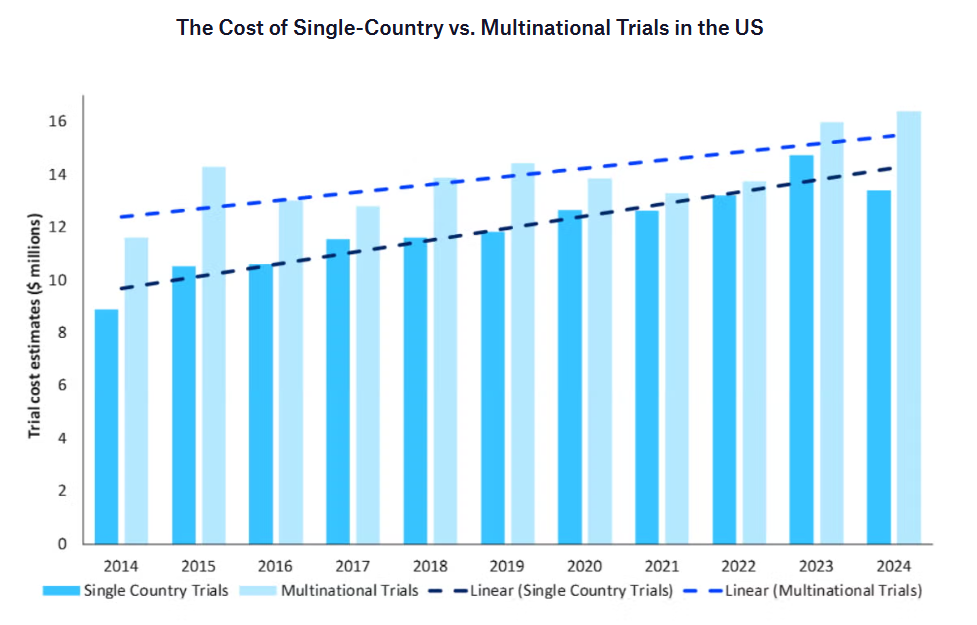
Source: Clinical Trials Arena
Analysts estimate that about $18 billion, or roughly 40% of current CRO value, could shift from costly labor-intensive work to automated AI solutions. The upside for CROs comes in three ways. First, productivity improvements powered by generative AI could create about $3 billion in value, as tools help prepare documents, manage data and speed routine workflows. Second, rethinking how trials operate altogether — including “clinical control towers” that track progress and optimize decisions in real time — could unlock nearly $9 billion in gains. Third, entirely new business models could add $7 to $10 billion in value, as CROs take on new roles integrating data providers, technology partners and trial sites to deliver end-to-end services.
AI also directly strengthens the science behind trials. New techniques such as self-supervised learning can analyze huge amounts of unlabeled data and uncover patterns humans would never spot. Foundation models — similar to large AI models like ChatGPT — can be trained specifically for drug development, helping predict which compounds are promising or how molecules might interact with cells. AI can even generate synthetic biological data or create “digital twins” of patients to simulate treatment outcomes. Early studies suggest AI-assisted trials have higher success rates, with 80% to 90% success in Phase I and around 40% in Phase II, compared with lower historical averages. For CROs, the message is simple: adopt AI to run trials faster, cheaper and with better outcomes.
What to Consider
The drug development landscape is transforming rapidly and facing challenges. CROs that embrace the above trends can support genomic-driven science, solve persistent trial bottlenecks and deploy digital and AI tools to speed execution, making them attractive to retail investors. Investors may consider:
- Companies expanding capabilities in genomics, biomarkers, and specialty therapeutics
- IQVIA (IQV)
- Medpace (MEDP)
- Charles River Laboratories (CRL)
- Illumina (ILMN)
- Thermo Fisher Scientific (THERM)
- Companies specializing in digital enrollment, remote monitoring, and flexible execution
- IQVIA (IQV)
- ICON plc (ICLR)
- Q2 Solutions & LabCorp (LAB)
- QIAgen (QIA)
- Medpace (MEDP)
- Companies using automation, analytics, or AI to cut cost and speed study timelines
- IQVIA (IQV)
- Medpace (MEDP)
- LabCorp (LAB)
- Illumina (ILMN)
- Thermo Fisher Scientific (TMO)
And you can get invested in these through a custom portfolio created by Bloom:
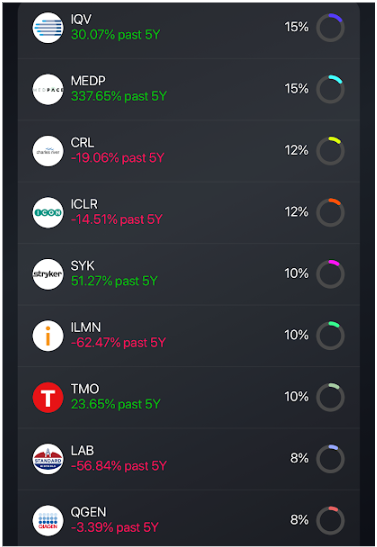
*QGEN is now QIA